Author : Rumana Reza
An empty cup could be poured by knowledge,otherwise nothing new could be injected there . So, let's start from 0/nil .......
- Particles of matter
- >Atoms
- >Molecules
-> Macromolecules
-> Cell organelles
-> Cells
-> Tissues
->Organs
-> Systems
-> Organisms
-> Populations
-> Ecosystems
-> Biomes
-> Planets
-> Planetary Systems with Stars
->Galaxies
->The Universe
->Infinity
- Particles of matter
- >Atoms
- >Molecules
-> Macromolecules
-> Cell organelles
-> Cells
-> Tissues
->Organs
-> Systems
-> Organisms
-> Populations
-> Ecosystems
-> Biomes
-> Planets
-> Planetary Systems with Stars
->Galaxies
->The Universe
->Infinity
Here we will focus on those things only which are relevant to understand the structural system of this universe.
 |
States of MatterLiterature ReviewAtom |
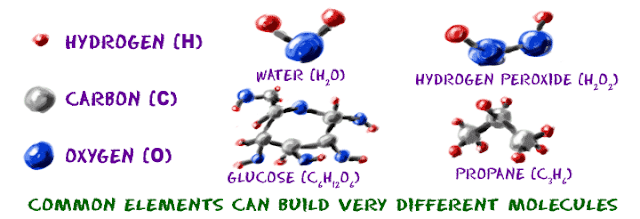
Atoms are building blocks. To build molecules, we will
need atoms of different elements. Atoms
are the general term used to describe pieces of matter. We have billions of
billions of atoms in our body. However, we may only find about 40 elements - billions
of hydrogen (H) atoms, billions of oxygen (O) atoms, and a bunch of others. All of the atoms are made of the same
basic pieces, but they are organized in different ways to make unique elements.
Bohr model
In 1913 the physicist Niels
Bohr proposed a model in which the electrons of an atom were assumed to
orbit the nucleus but could only do so in a finite set of orbits, and could
jump between these orbits only in discrete changes of energy corresponding to
absorption or radiation of a photon.
Later in the same
year Henry Moseley provided additional experimental evidence in favor
of Niels Bohr's theory. These results refined Ernest Rutherford's and Antonius
Van den Broek's model, which proposed that the atom contains in its nucleus a
number of positive nuclear charges that is equal to its (atomic) number in
the periodic table. Until these experiments, atomic number was not
known to be a physical and experimental quantity. That it is equal to the
atomic nuclear charge remains the accepted atomic model today.
In 1913 the physicist Niels
Bohr proposed a model in which the electrons of an atom were assumed to
orbit the nucleus but could only do so in a finite set of orbits, and could
jump between these orbits only in discrete changes of energy corresponding to
absorption or radiation of a photon.
Later in the same
year Henry Moseley provided additional experimental evidence in favor
of Niels Bohr's theory. These results refined Ernest Rutherford's and Antonius
Van den Broek's model, which proposed that the atom contains in its nucleus a
number of positive nuclear charges that is equal to its (atomic) number in
the periodic table. Until these experiments, atomic number was not
known to be a physical and experimental quantity. That it is equal to the
atomic nuclear charge remains the accepted atomic model today.
 |
| The Bohr model of the atom, with an electron making instantaneous quantum leaps from one orbit to another. This model is obsolete : Kurzon - Own work |
Chemical bonds between atoms were explained as the interactions between
their constituent electrons. As
the chemical properties of the elements were known to largely
repeat themselves according to the periodic law, in 1919 the American chemist Irving Langmuir suggested that this could be
explained if the electrons in an atom were connected or clustered in some
manner. Groups of electrons were thought to occupy a set of electron shells about the nucleus.
Atomic orbital
An atomic
orbital is a mathematical
function that describes the wave-like behavior of
either one electron or a pair of electrons in an atom. This
function can be used to calculate the probability of finding any electron of an
atom in any specific region around the atom's nucleus. The term may also
refer to the physical region or space where the electron can be calculated to
be present, as defined by the particular mathematical form of the orbital.
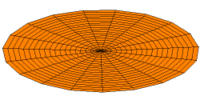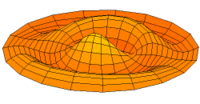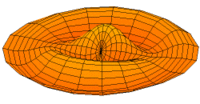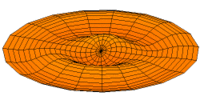
s-type modes
 |
Mode  (1s orbital) (1s orbital) |
 |
Mode 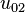 (2s orbital) (2s orbital) |
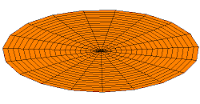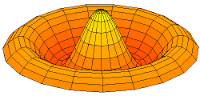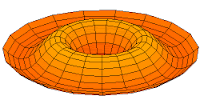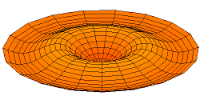 |
Mode  (3s orbital) (3s orbital) |
*********************************************************************
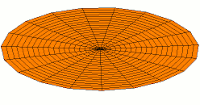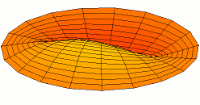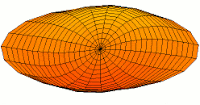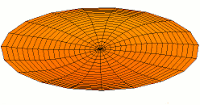 |
Mode  (2p orbital) (2p orbital) |
 |
Mode  (3p orbital) (3p orbital) |
 |
Mode  (4p orbital) (4p orbital) |
*********************************************************************************
d-type modes
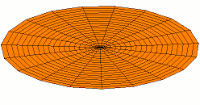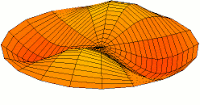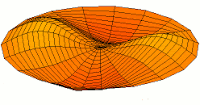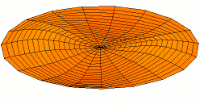 |
Mode 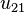 (3d orbital) (3d orbital) |
 |
Mode  (4d orbital) (4d orbital) |
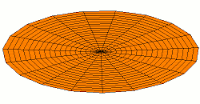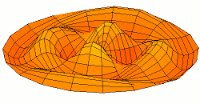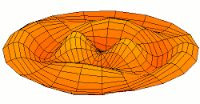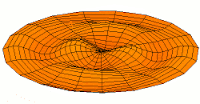 |
Mode  (5d orbital) (5d orbital) |
Quantum State
In quantum physics, quantum state refers to the
state of a quantum system.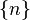 . For a more
complicated case, consider Bohm's formulation of the EPR experiment, where the state vector
. For a more
complicated case, consider Bohm's formulation of the EPR experiment, where the state vector
 . For a more
complicated case, consider Bohm's formulation of the EPR experiment, where the state vector
. For a more
complicated case, consider Bohm's formulation of the EPR experiment, where the state vector
involves superposition of
joint spin states for two
particles. Mathematically, a pure quantum state is represented by a state
vector in a Hilbert space over complex numbers, which is a generalization of our more usual
three-dimensional space. If this Hilbert space is represented as a function space, then its elements are called wave functions.
A mixed quantum state
corresponds to a probabilistic mixture of pure states; however, different
distributions of pure states can generate equivalent (i.e., physically
indistinguishable) mixed states. Mixed states are described by so-called density matrices. A pure state can also be recast as a density
matrix; in this way, pure states can be represented as a subset of the more
general mixed states.
For example, if the
spin of an electron is measured in any direction, e.g. with a Stern–Gerlach experiment,
there are two possible results: up or down. The Hilbert space for the
electron's spin is therefore two-dimensional. A pure state here is represented
by a two-dimensional complex vector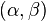
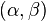
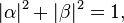
Before a particular measurement is performed on a quantum system, the theory usually gives only a probability distribution for the outcome, and the form that this distribution takes is completely determined by the quantum state and the observable describing the measurement. These probability distributions arise for both mixed states and pure states: it is impossible in quantum mechanics (unlike classical mechanics) to prepare a state in which all properties of the system are fixed and certain. This is exemplified by the uncertainty principle, and reflects a core difference between classical and quantum physics. Even in quantum theory, however, for every observable there are some states that have an exact and determined value for that observable.
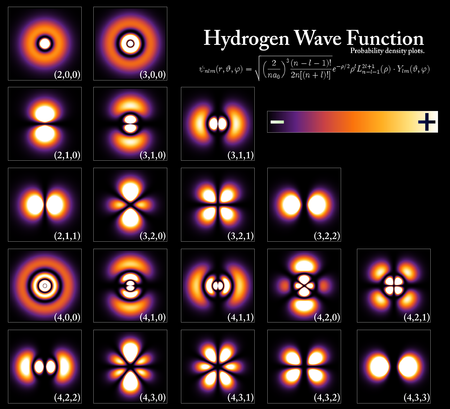 |
| Probability densities for the electron of a hydrogen atom in different quantum states.References: Forinash, Kyle. |
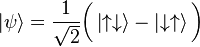
No comments:
Post a Comment